More than a third of US adults sleep less than recommended 7–8 h per night1,2. Insufficient sleep is associated with an increased cardiovascular risk, leading the American Heart Association to include sleep duration as the 8th metric of cardiovascular health in Life’s Essential 82,3,4. Female persons report sleep disturbances more frequently and have a more pronounced inflammatory response and cardiovascular risk associated with insufficient sleep than males2,4,5,6,7,8. We recently reported that randomly allocated mild, prolonged sleep restriction causes endothelial inflammation and dysfunction, early steps in the development of cardiovascular disease, in healthy female persons7. However, the underlying mechanisms remain unclear.
One suggested major function of healthy sleep is prevention of oxidative stress, an important contributor to endothelial inflammation and dysfunction9,10,11. Insufficient sleep, much like other cardiovascular risk factors, including cigarette smoking, hyperlipidemia, hypertension, and diabetes, generates intracellular oxidative stress11. Studies in Drosophila and rodent models have shown that sleep restriction increases oxidative stress (defined as increased generation of reactive oxygen species) and upregulates antioxidant response via induction of the antioxidant regulator nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a redox sensitive transcription factor that is kept in a latent state through its interaction with its repressor cullin-3 (Cul3)-containing ubiquitin ligase complex12,13,14. In response to increased oxidative stress, an adaptor protein Kelch-like ECH-associated protein 1 (Keap1) that binds to Nrf2 and Cul3 is modified and ubiquitin ligase complex is inactivated, allowing for Nrf2 accumulation and translocation into the nucleus where it binds to the antioxidant response element (ARE) and initiates the transcription of antioxidant genes15.
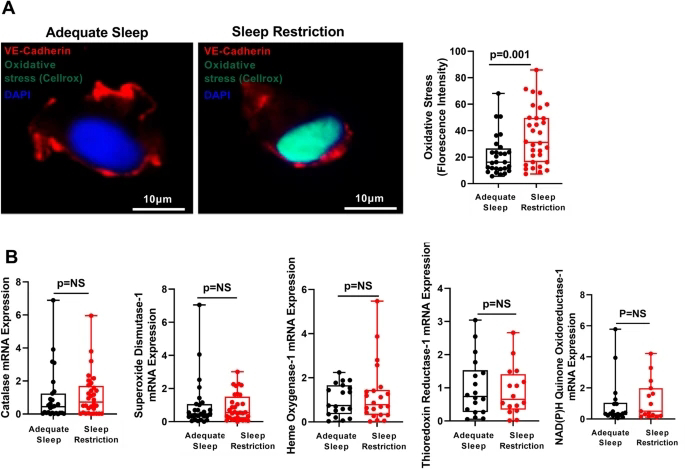
Organ-specific overexpression of antioxidant genes rescues the survival of severely sleep-deprived Drosophila11 and activation of the Nrf2-ARE pathway confers protection from cardiovascular diseases16, suggesting that intact antioxidant responses are essential to counteract detrimental effects of sleep restriction. Studies of the effects of insufficient sleep on oxidative stress in model organisms employed severe, acute sleep restriction or genetic manipulations that limit models’ lifespan11,17. Such extreme, short-term sleep curtailment has limited relevance to predominant populational sleep patterns of chronic, mild sleep curtailment owing to maintaining work/life balance in modern societies2,4,11,17,18. Whether chronic, mild sleep curtailment that mimics “real-life” sleep patterns affect endothelial oxidative stress and antioxidant responses is unknown. Using a randomized crossover design, we assessed oxidative stress and antioxidant responses directly in endothelial cells (ECs) freshly harvested from healthy female participants before and after objectively monitored 6 weeks of mild sleep restriction or adequate sleep.